Life Sciences
Expert Lifecycle Management for Life-Changing Products
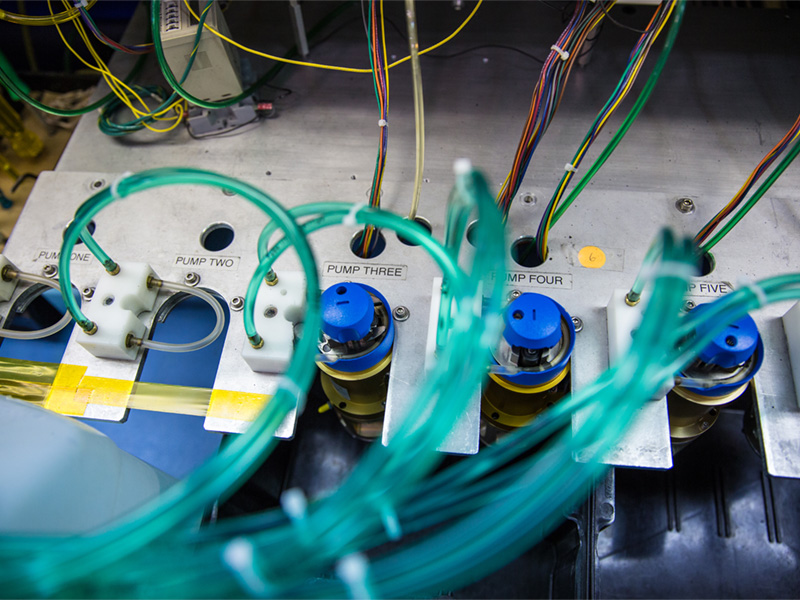
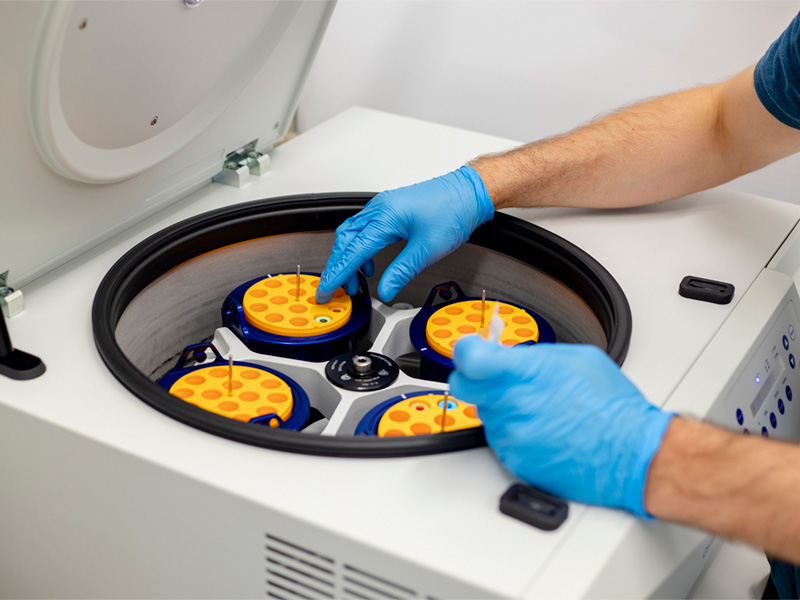
At Spartronics, we help the world’s most innovative healthcare and life-science companies develop and deliver sophisticated life-sustaining and critical-care devices and instrumentation — bettering the lives of people across the globe.
As a leader in full-service contract manufacturing and product-lifecycle management for the healthcare and life-science sector, we specialize in complex research, diagnostic and monitoring devices that meet the highest FDA standards of quality and traceability. Our customers rely on Spartronics for strategic and technical expertise, uncompromising quality, on-time delivery and dedicated customer support.
Centers of Excellence and Quality Certifications
The life-sustaining and critical-care devices we manufacture range in complexity through FDA Classes I, II & III. We operate FDA-registered facilities providing quality equipment with an integrated supply chain ensuring smooth logistics and on-time delivery. By meeting the highest regulatory and industry standards, we take pride in knowing that our work helps improve the outcomes and lives of patients.
- 21-CFR820
- FDA/QSR Registered Class I, II and III
- IEC 60601-1
- ISO 9001:2015
- ISO 13485:2016
- ISO Class 8
- RoHS and REACH
- MDSAP
Applications Overview
Partnership is at the core of everything we do for our healthcare and life science customers. We offer on-demand access to customer focused team, rapid responses, and regular status updates for smoother project management and peace of mind for various applications.
- Biopsy Systems
- Cancer Diagnostics
- Cardiovascular Diagnostics
- DNA Sequencing
- Immunoassay
- In Vitro Diagnostics
- Lab Automation
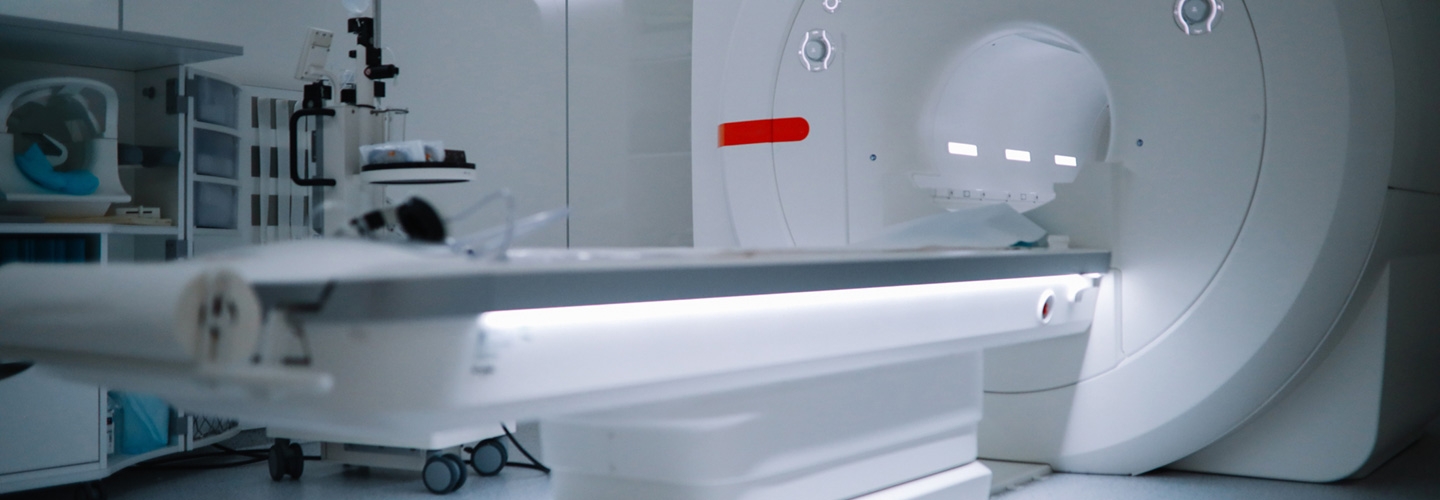
- Medical Imaging
- Molecular Diagnostics
- Ophthalmological Devices
- Point-of-Care Diagnostics
- Research Instrumentation
- Respiratory Therapy
- Therapeutic Devices
- Biopsy Systems
- Cancer Diagnostics
- Cardiovascular Diagnostics
- DNA Sequencing
- Immunoassay
- In Vitro Diagnostics
- Lab Automation
- Medical Imaging
- Molecular Diagnostics
- Ophthalmological Devices
- Point-of-Care Diagnostics
- Research Instrumentation
- Respiratory Therapy
- Therapeutic Devices
Product & Services Detail
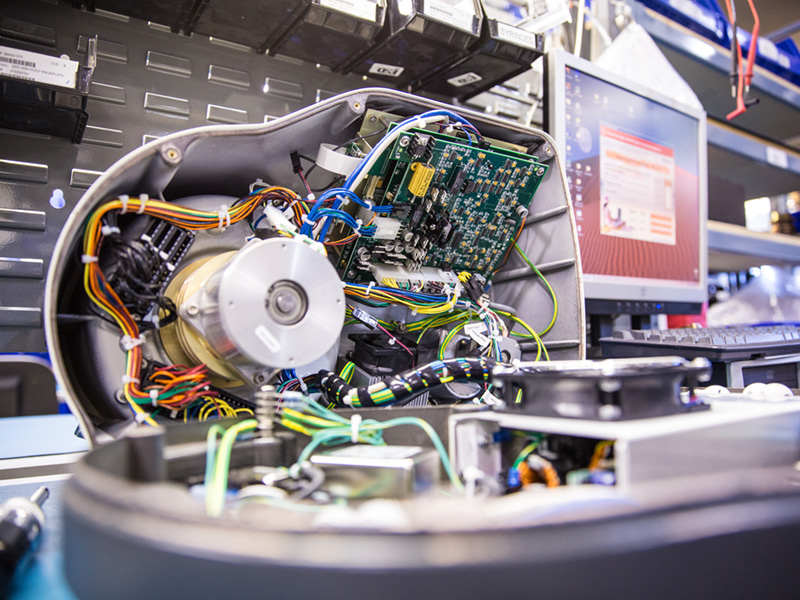
Our advanced manufacturing capabilities include printed circuit board assemblies (PCBA), sub-assemblies and finished products. Assemblies and sub-assemblies often also require specialized conformal coating, inspection and test coverage — to precision secondary operations. Finished life-sciences products often require additional services such as depot decontamination in repair, logistics services, loaner tool management and more.